
The Tangled Tree: A Radical New History of Life
This is delta H, Franch said.
20
Immediately after his epiphany, Woese shared it with George Fox, the postdoc he had assigned to work with Bill Balch on growing the methanogens. As recalled later by Fox, Woese “burst into my room in the adjoining lab” with the announcement that they had something unique. From there he proceeded throughout the lab, among his young students and assistants, “proclaiming that we had found a new form of life. He then pointed out,” by Fox’s memory, tart and amused, “that this was of course contingent on my having not screwed up the 16S rRNA isolation.” Being cautious, they repeated the whole process with delta H and got the same result. So no, Fox hadn’t screwed up.
“George was always skeptical,” Woese himself wrote later about their reactions to the discovery, adding that he valued such skepticism as good scientific instinct. Fox’s doctorate in chemical engineering suited him well to offset epiphanic leaps, even by the boss, with empirical caution. In fact, their shared instinct for skepticism about such a startling result helps explain why these two men worked so well together. But the anomalies in the fingerprints persuaded Fox too. By his account, they seemed to “jump off the page,” and he agreed that those differences suggested a third, very distinct form of life.
Still, Woese and Fox both knew that convincing other scientists of such an epochal discovery would be difficult. More data were needed. So the Woese lab went back to work, with Balch’s methodology and help, on culturing and fingerprinting still another methanogen. Woese and his colleagues worked quietly, for the time being. By the end of 1976, they had five additional genetic catalogs from five more methanogenic microbes, all quite different from one another but sharing signs of a much greater, much deeper, and shared difference from anything else known to exist.
21
Bacteria are versatile and diverse. That’s an understatement. Widespread—another understatement. They are hard to categorize, hard to identify, hard to sort into related groups, as even Stanier and van Niel finally admitted. They are nearly ubiquitous across most parts of Earth’s expanse, in both natural and human-made environments, floating through the air, coating surfaces everywhere, awash in the oceans, even present in rocks deep underground. Your skin as I’ve said is covered with them. Your gut is teeming. Your human cells may be outnumbered by them at a three-to-one ratio in your body. Bacteria live also in mudholes and hot springs and puddles and deserts, atop mountains, deep in mines and caves, on the tabletops at your favorite restaurant, and in the mouths of you and your dog.
A species called Bacillus infernus has been cultured from core samples of Triassic siltstone, buried strata at least 140 million years old, drilled up from almost two miles beneath eastern Virginia. Under the Pacific Ocean, 35,755 feet deep in the Mariana Trench, lie sediments that have also yielded living bacteria. In Antarctica, a body of water known as Subglacial Lake Whillans, lidded by half a mile’s thickness of ice and supercooled to just below zero, harbors a robust community of bacteria. They thrive there in the darkness and cold, eating sulphur and iron compounds from crushed rock.
Then again, some like it hot. Those are called thermophiles. Among the most famous of thermophilic bacteria is Thermus aquaticus, first cultured from a sample collected in Yellowstone National Park by the microbiologist Thomas Brock and a student, Hudson Freeze, in 1966. Brock and Freeze had found it in a steaming, multicolored pool called Mushroom Spring, in Yellowstone’s Norris Geyser Basin, at a temperature of about 156 degrees Fahrenheit. Functioning in such heat, Thermus aquaticus contains a specialized enzyme for copying its DNA, one that performs well at high temperatures, which became a key element in the polymerase chain reaction technique for amplifying DNA. That technique, widely useful in many aspects of genetic research and biotech engineering, earned its chief developer (but not Thomas Brock) a Nobel Prize.
Other heat-loving bacteria can be found around hydrothermal vents on the sea bottom, where they help anchor the food chains, producing their own organic material from dissolved sulfur compounds vented out with the hot water, and being fed upon by little crustaceans and other animals. A giant tube worm, one of those gaudy red creatures that waggle around such vents, with no mouth, no digestive tract, gets its nutrition from bacteria growing within its tissues.
By one estimate, the total mass of bacteria exceeds the total mass of all plants and animals on Earth. They have been around, in one form or another, for at least three and a half billion years, strongly affecting the biochemical conditions in which most other living creatures have evolved. That we don’t see bacteria is simply because our eyes are not calibrated to the appropriate scale. There may be more than a billion bacterial cells in an average ounce of soil, and five million in a teaspoon of fresh water, but we can’t hear their crackle or their fizz. A single kind of marine bacteria known as Prochlorococcus marinus, which drifts free in the world’s tropical oceans and photosynthesizes like a plant, may be the most abundant creature on Earth. One source places its standing population at three octillion individuals, a number that looks like this: 3,000,000,000,000,000,000,000,000,000.
They vary in shape and in size—interestingly in shape, drastically in size. A bacterial cell, on average, is about one-tenth as big as an animal cell. At the upper end of the range is Thiomargarita namibiensis, an odd thing discovered on the sea floor near Namibia, its cells ballooning up to three-quarters of a millimeter in diameter, stuffed with pearly globules of sulfur. At the lower end of the range is Mycoplasma hominis, a tiny bacterium with a tiny genome and no cell wall, which manages nonetheless to invade human cells and cause urogenital infections.
Bacterial shapes, as I’ve mentioned, range through rods, spheres, filaments, and spirals, with variations that in some cases represent adaptations for movement or penetration. It turns out that their geometries, notwithstanding the efforts and convictions of Ferdinand Cohn, are unreliable guides to their phylogeny. Shape can be adaptive, but adaptations can be convergent as well as ancestral. Roundness may be good as a hedge against desiccation. Elongation as a rod or filament seems to help with swimming, and a flagella definitely does. Filamentous bacteria that are star shaped in cross section, recently discovered in a wonderfully named substance called “mine-slime,” deep in a South African platinum mine, may profit from all their surface area by way of enhanced absorption in nutrient-poor environments. The twisting motion of spirochetes, such as the ones that cause syphilis and Lyme disease, evidently allows them to wiggle through obstacles that other bacteria can’t easily cross, such as human organ linings, mucous membranes, and the barrier between our circulatory system and our central nervous system—a fateful degree of access. Even the less dynamic shapes, the short rods known as bacilli, the spheres known as cocci, and the rods slightly curved like commas, serve well enough the bacteria responsible for a long list of diseases: anthrax, pneumonia, cholera, dysentery, hemoglobinuria, blepharitis, strep throat, scarlet fever, and acne, among others.
Although many bacteria live as solitary cells, taking their chances and meeting their needs independently, others aggregate into pairs, clusters, little scrums, chains, and colonies. The coccoid cells of Neisseria gonorrhoeae, which cause gonorrhea, lump together by twos, forming bilobed units resembling coffee beans. The genus Staphylococcus gets its name from Greek words for “granule” (kókkos, the spheroid aspect) and “a bunch of grapes” (staphylè), because staph cells tend to bunch. Most of the forty staph species are harmless, but Staphylococcus aureus can inflict skin infections, sinus infections, wound infections, blood infections, meningitis, toxic shock syndrome, plus other nasty conditions, and if you’re so unlucky as to pick up a dose of those little grapes in one of their antibiotic-resistant forms, such as MRSA, a monstrous product of horizontal gene transfer (as I’ve mentioned, and to which I’ll return), you could be in a world of hurt. Cells of Streptococcus species, including those that cause impetigo and rheumatic fever, stick together like beads on a chain.
Bacteria can also form stubborn, complex films on certain surfaces—the rocks of a sea floor, the glass wall of an aquarium, the metal ball of your new artificial hip—where they may cooperate together in exuding a slimy extracellular substance that helps nurture them collectively, maintain the stability of their little environment, serve as a sort of communications matrix among them, and even protect them from antibiotics. These living slicks, known as biofilms, can be thinner than tissue paper or as thick as a good dump of snow, and may incorporate multiple species. The little rods of Acinetobacter baumannii are infamous for their ability to lay down persistent biofilms on dry, seemingly clean surfaces in hospitals.
Cyanobacteria, including that monumentally abundant Prochlorococcus, convert light to energy and deliver, as byproduct, a large share of Earth’s free atmospheric oxygen. Purple bacteria photosynthesize too, but do it by drawing upon sulfur or hydrogen instead of water as fuel for the process, and they don’t produce oxygen. Lithotrophic bacteria, the rock eaters, deriving their energy from iron, sulfur, and other inorganic compounds, exist in more ingenious variants than you care to know. Japanese researchers have recently discovered a new bacterium, Ideonella sakaiensis, that digests plastic. Certain enterprising ocean bacteria, such as Marinobacter salarius, have risen to the challenge of degrading hydrocarbons from the Deepwater Horizon oil spill. Other bacteria are quite capable, in the presence of oxygen or without it, of feasting on garbage, sewage, various inorganic compounds, plants, fungi, and animal tissue, including human flesh. Lactic acid bacteria, which may be rod shaped or spherical, turn up in milk products, busy at their task of carbohydrate fermentation and resistant to the acid they create. Many of them also like beer.
Not all such particulars were known to Carl Woese in 1977 as he examined the fingerprints from his first few methanogens. But the vast scope, ubiquity, and multifariousness of bacteria certainly were. The terrain of bacteriology was known even better to Ralph Wolfe, who had trained in the classic fundamentals under van Niel and others. Woese’s reaction to his own preliminary results must have seemed all the more radical, then, all the more shocking, as he shared it not just with George Fox and members of his own lab but also with Wolfe, just after they repeated the rRNA analysis of delta H, the first methanogen. “Carl’s voice was full of disbelief,” Wolfe wrote in a memoir, “when he said, ‘Wolfe, these things aren’t even bacteria.’”
22
Ralph Wolfe told me the same story, with some elaboration, thirty-nine years later when I called on him in Urbana. By then, he was an emeritus professor of microbiology, ninety-three years old, a frail and slender gentleman with a quick smile, still maintaining his office and coming to it, as though retirement were not an entirely satisfying option. On the wall behind his desk hung a replica of Alessandro Volta’s pistola, a gunlike device invented by Volta in the late 1770s for testing the flammability of swamp gases, including methane. On the desk itself were papers and books and a computer.
Woese’s lab back in the day had been in Morrill Hall, on South Goodwin Avenue, and Wolfe’s was in an adjacent building, connected by a walkway. Woese would occasionally trundle over on various business. “He came down the hall and happened to see me,” Wolfe recalled, “and says, ‘Wolfe, these things aren’t even bacteria!’” Wolfe laughed gently and, for my benefit, continued reenacting the scene.
“‘Of course they are, Carl.’” They look like bacteria in the microscope, Wolfe had told him. But Woese wasn’t using a microscope. He never did. He was using ribosomal RNA fingerprints.
“‘Well, they’re not related to anything I’ve seen.’” Coming back to the present, Wolfe said: “That was the pivotal statement that changed everything.”
23
We went into fast-forward mode,” Woese recalled in his account of these events. By the end of 1976, his team had done fingerprints and catalogs on five additional methanogens, with more in the pipeline. And sure enough, he wrote, none of the new catalogs was prokaryotic, not in the prevailing sense of that word, which meant bacteria and only bacteria. None of the organisms was eukaryotic, either. But “they were all of a kind!”—a third kind, something else, something anomalous, something hitherto unsuspected to exist. Woese started thinking that he would need to declare a new kingdom of life—create a new name, invent a huge new category—to recognize their uniqueness and contain them. It wasn’t really a new kingdom, of course. It was a newly discovered natural grouping of life-forms, which had existed apart for a long time, unrecognized, and which might be called a “kingdom” or an “urkingdom” or a “domain,” according to preferred human convention.
Woese believed that this discovery, still unannounced, offered “a rare opportunity to put the theory of evolution to serious predictive test.” He meant Darwin’s theory of evolution, as opposed to any others—the one that recognized hereditary continuity plus a degree of random variation over long stretches of time, and explained the shaping of that variation, to yield adaptation and diversity, mainly by way of natural selection. If Woese’s preliminary findings were correct, he noted, those findings should serve as a guide for predicting roughly what further data and discoveries would appear. From the premise that 16S rRNA represented a very slow-ticking molecular clock, with a minimum of selected variation, he deduced that his newly found kingdom must represent a very old division. Very old—having originated near the beginning of cellular life, maybe three and a half billion years ago. Now he would try to sketch its boundaries and its characteristics. As he and his team added more microbes to its membership—more methanogens and maybe other creatures too, each known by its catalog of RNA fragments—Woese expected two things: that this unnamed kingdom would remain dramatically distinct from the rest of the living world and that it would nonetheless encompass great diversity. “Testing these two main evolutionary predictions,” he wrote, “drove our work from that point on.”
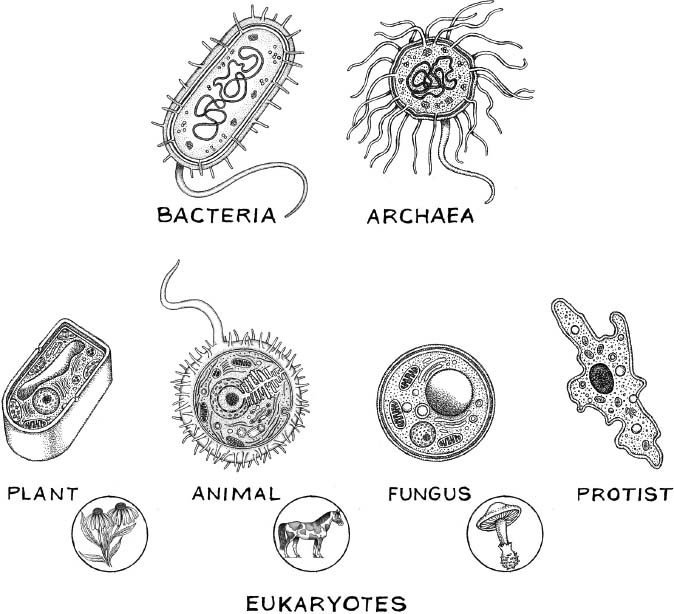
Three domains and (within the eukaryotes) four kingdoms, four types of cell.
In August the team published a carefully limited paper, just a hint of what was coming, in the Journal of Molecular Evolution, the same journal at which Emile Zuckerkandl continued to serve as editor. It was a logical match of subject and outlet because Zuckerkandl, back in his days as Linus Pauling’s sidekick, had helped articulate the very premise that Carl Woese was now putting dramatically to use: that the branching of lineages “should in principle be definable in terms of molecular information alone.” The molecular information at issue in this case consisted of ribosomal RNA sequences from the first two methanogens Woese’s team had characterized. One of those methanogens was a strain of M. ruminantium, isolated from rumen fluid (from the paunch of a cow) donated by a friendly contact in the university’s Department of Dairy Science. The other was delta H, the conveniently nicknamed strain of the fourteen-syllable monstrosity, M. thermoautotrophicum, known to live at high temperatures and metabolize hydrogen. I asked Ralph Wolfe where they had gotten their starter sample of that exotic beast, delta H.
“It was isolated here from the sewage.” More specifically, from a sewage sludge digester.
“In Urbana?”
“Yeah.”
The first author on this discreet paper was Bill Balch, Wolfe’s graduate student, who had earned his authorship priority by developing the sealed-tube technique of growing and labeling methanogens. “It was because of that technique,” Wolfe told me, “that we could now do these experiments with Carl. Because everything was sealed, and you could now inject the P-32 into the culture.” P-32, remember, was the radioactive phosphorus. “Whereas the previous techniques, you had to keep opening the stopper and flushing it out, and it would have been a radioactive nightmare to do it that way.” Balch’s system allowed for injecting the P-32 by syringe through the black rubber stopper. Balch grew the microbes, George Fox extracted the RNA, and Woese’s trusted lab technician at the time, a young woman named Linda Magrum (she had replaced the earlier Linda in that role, Linda Bonen), prepared the fingerprint films for Woese to analyze. All three of them, plus Ralph Wolfe himself, appeared as coauthors, with Woese’s name last, reflecting his role as senior author. Besides describing the methodology, this paper noted drily that the two methanogens didn’t look much like “typical” bacteria. It mentioned that the divergence might represent “the most ancient phylogenetic event yet detected”—a big claim, vague enough as stated to pass almost unnoticed.
In October the team published a second paper, in a more far-reaching journal, the Proceedings of the National Academy of Sciences (known as PNAS). This time George Fox was first author, and the data covered ten species of methanogen, each one assessed for similarity to the other nine and to three species of what the authors still cautiously called “typical bacteria.” Fox had created a simple measurement system by which the catalog of one microbe could be compared with the catalog of another, yielding a decimal number—a coefficient—representing degree of similarity. Comparing each of these thirteen microbes with all the others gave an overall picture of which were how closely similar to which others. The data could be arranged in a rectangular table, names down the left margin, names again across the top, numbers at each cross point, as in a chart showing the various mileage distances between all pairs in a list of cities. Instead of mileage: a similarity coefficient. From those numbers, and the premise that similarity reflected relatedness, Fox generated a dendrogram, a branching figure, showing nodes of divergence between major lineages and a branch for each organism. Although they printed this dendrogram sideways—like a bracket for the NCAA basketball tournament—rather than vertically, it was, in fact, a tree: the first of the new trees of life in the era of Carl Woese. There would be many more.
This one showed the “typical bacteria” occupying one major limb. The ten methanogens all branched from a second major limb. “These organisms,” said the paper, “appear to be only distantly related to typical bacteria.” Again the five authors were saying less than what they believed. The phrase “typical bacteria” was an interim delicacy that would soon disappear.
A third paper, the most bold and dramatic, appeared in PNAS a month later under the authorship of Woese and Fox alone. Its title hinted only obliquely at its intent: to reorganize “the primary kingdoms” of life. Again using Fox’s similarity coefficients, it compared methanogens against one another and against “typical bacteria,” and each of those also against several eukaryotic organisms, including a plant and a fungus. Its conclusion was radical: there are three major limbs on the tree of life, not two. The prokaryote-eukaryote dichotomy, as proposed by Stanier and van Niel, as generally accepted throughout biology, is invalid. “There exists a third kingdom,” Woese and Fox wrote, and it includes—but may not be limited to—the methanogens. It isn’t the bacteria, and it isn’t the eukaryotes, they explained. It’s a separate form of life.
The two authors gave their kingdom a tentative name: archaebacteria. Archae- seemed apt, suggesting archaic, because the methanogens appeared so ancient, and their metabolism might have been well suited to early environments on Earth, about four billion years ago, before the onset of an oxygen-rich atmosphere. Woese had made that very point in an interview with the Washington Post. “These organisms love an atmosphere of hydrogen and carbon dioxide,” he said (or at least, so he was quoted). “Just like the primitive earth was thought to be,” he said, adding, “No oxygen and very warm.” But the other half of that compound label, archaebacteria, tended to blur the central point of the discovery: that, as Woese had announced to Wolfe, these things aren’t even bacterial forms of life. They’re quite different. Wolfe himself told Woese that archaebacteria was a terrible choice. If they’re not bacteria, why retain that word at all? The provisional name stuck for about a dozen years, before being emended to something better, something that stood by itself: the archaea.
24
George Fox was no longer a rangy young man when I sat with him in a nondescript pizza parlor near the campus in Urbana, after the opening session of a Carl Woese memorial symposium, and watched him eat a nondescript little pizza. Fox is a man who prefers simple, plain food, and he had cringed when I ordered pepperoni and mushrooms on my own. At age sixty-nine, he carried the full body and slight jowls of a lifetime spent in laboratories and classrooms; wire-rim spectacles had replaced the dark horn-rimmed glasses he had worn in the 1970s photos, and his brown hair was graying at the temples, but his eyes still shined brightly blue as he recalled the days and years with Woese. Now a professor at the University of Houston, Fox had flown up for the Woese meeting, which was hosted by the Carl R. Woese Institute for Genomic Biology (its name reflecting the fact that Woese has become a venerated brand at the University of Illinois). Fox would give one of the invited talks.
He had spent his academic career at three institutions: Houston, for almost three decades; preceded by Illinois, as a postdoc with Woese; and before that it was Syracuse University, as an undergraduate and PhD student. The circumstances of Fox’s arrival in Urbana were haphazard, beginning from a coincidence in Syracuse, where Woese himself grew up. There at the university, Fox belonged to a professional engineering fraternity, Theta Tau, of which Carl Woese’s father—also named Carl Woese—was a founder, and so Fox was required to know the name. As he shifted interest from chemical engineering to theoretical biology, he noticed and became fascinated by some of the early work of Carl Woese the son. In particular, there was a paper on what Woese called a “ratchet” mechanism of protein production by ribosomes—a risky proposal, a wild and interesting idea (later proven wrong in its details), published in 1970. So Fox wrote to this ratchet guy asking for a postdoc fellowship, and Woese seemed to see the Syracuse connection as karma. He had a position to fill, yes, with the departure of Mitch Sogin, the ultimate handyman grad student, and he offered that to Fox.

