
The Open Sea: The World of Plankton
FIG. 16
The Secchi disc for measuring the transparency of the sea.
In passing I may say that the Secchi disc is also valuable in helping us to compare the varying colour of the water. Here, of course, I am not thinking of the often striking and delightful changes of hue which we may see as light of different quality is reflected from a changing sky: when for example dark grey clouds give place to an open space of blue or when cumulus clouds dapple the sea with purple shadows. Reference was made in Chapter 2 to the contrast between the green water of the Channel and the deep blue of the Atlantic; such differences are due to the nature of the contents of the sea itself and are examples of what I mean by the varying colour of the water. A great variety of shades and hues may be found at different times; these are mainly due to the presence of different kinds of very small plankton organisms in exceptional numbers. The light reflected back through the water from the white background of the disc enables us to judge and compare these differences more easily than by just looking into the depths. Dense concentrations of diatoms such as Rhizosolenia and Biddulphia, or of the colonial flagellate Phaeocystis, may give a brown appearance to the water over large areas; the North Sea fishermen often call such patches of water “Dutchman’s baccy juice”. Some dinoflagellates may make the water almost red, coccolithophores may give it the white milky appearance referred to in the last chapter, and other small flagellates may occasionally make it a vivid green.
But let us return to the sunlight and these little plants of the sea; in order to flourish and grow they must produce more oxygen in the process of photosynthesis (see here) than they use up in respiration. Plants, of course, breathe as well as animals. Some very significant experiments were performed in the Clyde sea-area by Drs. Marshall and Orr (1928) of the marine biological station at Millport on the Island of Cumbrae. They grew cultures of diatoms in glass bottles in the sea at different depths; these they suspended on strings from a long thin rod between two buoys at the surface and so kept them free of shadow. All their bottles were in pairs; one of each pair was exposed to the light and the other covered with a black cloth. In each bottle the oxygen-content of the water was measured at the beginning of the experiment and again at the end of twenty-four hours. An increase in the oxygen in the uncovered bottles showed the amount produced by photosynthesis less that used up in respiration; a fall in oxygen-content in the ‘blacked-out’ bottles measured respiration alone. By adding this oxygen-loss to the oxygen measured in the uncovered bottles the total oxygen-production as a record of photosynthesis could be estimated. The experiments were repeated as the spring passed into summer, and were also made on days which were overcast and on others which were sunny. As the sun went higher in the sky and the light became more intense the depth at which diatoms could produce more oxygen than they used in respiration increased from a depth of less than 10 metres on an overcast day in March to nearly 30 metres on a sunny day at midsummer. By far the greatest photosynthetic activity—on which their growth depends—took place, however, in the top 5 metres. In the waters round Great Britain we may now say that practically all the plant-production that matters takes place in the top 10 or 15 metres. This is one important clue in the puzzle of the seasons; we must now turn to temperature.
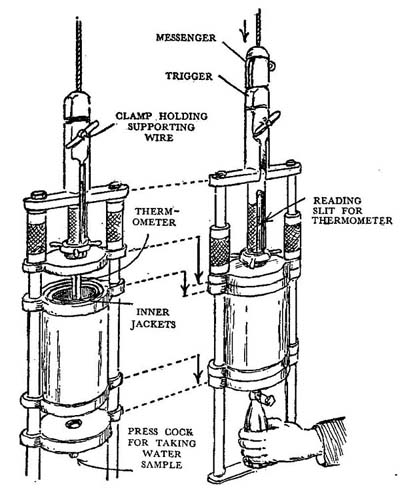
FIG. 17
The Nansen-Pettersen water sampling bottle: shown open and closed.
The water round our coasts varies in temperature from about 8°C in winter to sometimes as much as 17°C in the Channel in a warm summer. It is, of course, because the sea loses and gains heat so much more slowly than the land that we in Britain have so equitable a climate compared to that of an area in the middle of a continent. Two methods are used in taking the temperature of the sea. Down to moderate depths, say to 50 metres, the insulated Nansen-Petterson water-bottle, which is shown in Fig. 17 is used; it is of metal and is sent down suspended on a wire to obtain samples of water both for chemical analysis and for temperature determination. It goes down with the bottom and top open so that water can circulate through it; then at the required depth a small ‘messenger’ weight is sent sliding down the wire to hit a trigger which releases springs to close it. Projecting through the top, in a protective casing, is the stem of a thermometer whose bulb is in the centre of the sampling bottle; its scale and mercury thread are visible through a slit in the upper casing so that it can be read as soon as the bottle is brought back to the surface. There are actually three walls to the cylindrical bottle, one inside the other, with a little space between; when the top and bottom are firmly closed there are thus two water jackets outside the bottle proper and these act as insulating chambers preventing loss or gain of heat in the water sample while it is coming up and the thermometer is being read. As soon as the temperature has been noted the water is run out from a cock at the bottom to be stored for later analysis and the bottle is opened ready to be sent down to another level. From much greater depths the bottle would take so long being drawn up that the insulation just described would not be adequate to prevent a change of temperature in the process. To get over this, special so-called reversing thermometers and bottles have been devised. The mercury tube of the thermometer, just above the bulb, has a loop and a kink in it, so that when it is swung rapidly upside down the thread of mercury breaks; as soon as this happens all the mercury that before was above the kink now runs to the opposite, and now lower, end of the tube. When it is brought up the height of this inverted column of mercury is seen against a scale which can only be read when the thermometer is upside down; it tells us the temperature that the thermometer was recording at the moment it was turned over. The bottle and thermometers (there are usually two to give check readings) are mounted in a frame which rotates when a trigger is hit by a messenger weight; the bottle, which before was open, is closed as it swings over.1
After this digression on thermometers, let us return to consider the temperatures of our seas with the passing of the seasons. Water, above 4°C, expands when warmed and contracts when cooled; so its density is altered: a given volume of cold water weighing more than the same volume of warm water. In winter the atmosphere is colder than the sea so that the surface waters are cooled and therefore sink beneath the warmer and less dense layers which were below; this is repeated again and again until after a time there is an almost uniform low temperature from top to bottom. The winter gales help in the process of mixing up the layers too. The sea, of course, is rarely so cold in winter or so warm in summer as is the atmosphere; as we have already noted, it gains and loses heat much more slowly. As spring passes into summer the air warms up and the radiant heat of the sun gets stronger, so we find the upper layers of the sea becoming warmer too; as they heat up they become increasingly lighter than the layers below and thus tend more and more to remain separated on the top because less and less are they likely to be mixed with the heavier waters beneath. This division between the upper and lower waters is called a discontinuity layer (or thermocline in still more technical language) and is usually set up at a depth of round about 15 metres. Let us take an actual example from the summer temperatures in the English Channel in July as found by the hydrologists of the Plymouth Laboratory. At depths from just below the surface down to 15 metres the temperature only varied from 16.5° to 15.82°C; but at 17½ metres it had dropped to 12.09°C and then, as it was sampled deeper and deeper, it remained practically constant to read 12.03°C at 60 metres. The upper layer was effectively cut off from the lower by this sudden drop in temperature of nearly 4°. A strong summer gale may destroy this discontinuity layer, but if it is not too late in the season it will soon form again. It is in the autumn that the air cools again and so the surface water loses heat; also the equinoctial gales stir up the sea and the more uniform temperatures of winter again become established from top to bottom. It will be noted that this warm summer upper layer corresponds very closely to the region (sometimes called the photic zone) in which the little plants get sufficient light to carry out effective photosynthesis. Two bits of the puzzle seem as if they would fit together; we require, however, yet another piece to go with them before we can see the explanation of the seasonal changes in the plankton. This last link concerns certain salts in the sea, and to them we must now turn.
First we must consider the general saltness of the sea; this, of course, is mainly due to the abundant sodium chloride which accounts for almost 77.8% of the total salt content. However there are many other salt constituents, of which the next more important, in order of descending quantity, are magnesium chloride (10.9%, magnesium sulphate (4.7%), calcium sulphate (3.6%, potassium sulphate (2.5%), calcium carbonate (0.3%) and magnesium bromide (0.2%). These proportions are actually those in which these different salts would be recovered from the sea on evaporation; their molecules as dissolved in the sea, however, would largely—some nine out of ten—be split up into their respective parts or ions: sodium and chlorine or magnesium and sulphate ions as the case may be. It is better to think of the salt constituents of sea water, as they mostly are in the sea itself, in terms of separate ions. We can tabulate the percentage proportions as follows, based upon a mean of 77 samples collected from different localities by the Challenger Expedition:

In addition there are minor constituents, for example iron, strontium, silicates, phosphates and nitrates, which constitute together only 0.06%. The degree of saltness of the sea, or its salinity, is usually expressed in terms of the total weight of salts in grams per thousand (°/oo) grams of sea-water; it varies in the open ocean from 34°/00 in polar waters, where it is low on account of additions of fresh-water from melting ice, to 37°/00 near the equator where it is high because of excessive evaporation of water. The North Atlantic surface water as it flows round our islands has a salinity of about 35°/00, but in the southern North Sea it is diluted to some 34.5°/00 by water-drainage from the land.
Now the important salts for our little plants are those which have only been mentioned among the minor constituents: they are the phosphates and the nitrates. Because they are present in such small quantities, it was a long time before accurate methods for their estimation could be devised; these were developed largely through the work of Drs. Atkins and Harvey at the Plymouth Laboratory just after the first world war. It had been realised that the plants of the sea must be limited, as are the plants of the land, according to Liebeg’s Minimum Law; i.e. so long as any really essential nutritive substance occurs in minimum quantities, plant production will be proportionate to the available quantities of it, even though there is a super-abundance of all other essentials. This seemed obvious enough but could not be proved until we had these more refined methods. It now became possible to measure the amounts of phosphates and nitrates taken up from the water by the little plants; it was shown that in our waters these salts could and did in fact limit their growth. The reproductive rate of these little plants grown in culture solutions was seen to fall off as the phosphates and nitrates were depleted and finally growth would stop altogether when they were entirely used up.
It is now possible to explain the seasonal cycle of events. In the winter, as we have seen, the waters from top to bottom are well mixed and their temperature is almost uniform. As the length of the days and the intensity of the light increases there comes a point at which the little plants can begin to multiply and they find a comparatively rich supply of phosphates available—about 40 milligrams per cubic metre of water. We have seen how rapidly they undergo fission when once they start. They are multiplying only in the upper, well illuminated zone; in the early spring these upper waters are being well mixed up with the lower layers by the equinoctial gales and there is a general reduction of the free phosphates as they pass into the plants. Presently, however, there develops an upper warm layer which becomes more pronounced as spring advances into summer; this is also the photic zone, in which alone the plants can multiply. The phosphates and nitrates are now being used up by the plants in this upper zone and are not being replaced by any mixing with the lower waters because of the difference in density between them. In fact the phosphates and nitrates are continually passing from the upper to the lower layers. The plants, which have taken up the salts, may either eventually form resting spores and sink, or may just die and sink, or more likely be eaten by the animal members of the plankton; these may themselves just die and sink or in turn be eaten by other larger animals and so on. The nutritive salts which were once present in the upper zones are now by late summer reduced to a minimum; they are carried in the falling bodies to the bottom or still locked up in animal life. Actually a good deal may be excreted back into the water by the animals,2 but as most of the animals only make comparatively short visits from the lower into the upper layers to feed on the plants at night, most of the excreted phosphates and nitrates will pass into the lower waters. Thus we see that so long as the discontinuity layer lasts, the plants, such as have not been eaten by the animals, are cut off from the richer phosphates and nitrates below. That is why their numbers decline so markedly as the summer advances and why they cannot reproduce at a rate sufficient to counterbalance the inroads made upon their population by the grazing animals. Down below, the supplies of phosphates and nitrates are being to some extent built up again by their return from dead animals broken down by bacterial action. Now, as the summer wanes, the upper layers are cooled again and the autumn equinoctial gales assist in a general mixing; the water richer in phosphates and nitrates is brought up from below towards the surface where once again we have a fertile layer while the sunlight is still strong enough to encourage photosynthesis.
Here at last we have the explanation of that autumnal outburst of phytoplankton which had for so long been such a puzzle. The time of its appearance and the quantity produced vary markedly in different years; it is usually not very long-lived and eventually the population of plants dwindles to a winter minimum as the light gets too weak to allow much active reproduction. The winter gales stir up the water and the nutrient salts are once again more or less evenly spread through the different layers of water. The temperature, too, is more or less uniform; the cycle is complete.
This brief account of the events throughout the year has dealt with the phytoplankton as a whole. If it suggests, as well it might, that all the different kinds of little plankton plants are increasing and declining together, as the seasons come and go, it would be giving a very false picture. There is in truth a succession of different forms which wax and wane in turn within this larger framework. As the summer advances and the quantity of the phytoplankton is declining, the dinoflagellates come to occupy a much more prominent part in the community; in late August species of Ceratium and Peridinium may be much more evident in the fine net samples than the diatoms. At the second autumn outburst the diatoms will swing back into prominence again. Then within these spring and autumn periods of production there is usually a fairly definite order of appearance of different species of diatoms as the weeks go by; not that one kind disappears entirely of course, but after a period of abundance the reproduction falls to a low ebb and the stock is maintained by only a few individuals or by the resting spores already referred to. The intensive work, already referred to (see here), carried on week by week for fourteen years at Port Erin in the Isle of Man, has furnished us with a mine of information about these detailed seasonal changes at one place; and now the monthly plankton recorder surveys which will be described in the last chapter (see here) are giving us similar information for a very wide area.
What makes one species give place to another? Why for example should Chaetoceros decipiens give way to Ch. debilis and socialis as the season advances or Rhizosolenia semispina be replaced by Rh. shrubsolei which in turn may leave the stage to Rh. stolterfothii? Whilst the grazing of the little plankton animals coupled with the reduction of phosphates and nitrates in the upper layers is bringing about the general decline in the planktonic vegetation it can hardly be controlling the rise and fall of the different species. Johnstone, Scott and Chadwick (1924) in discussing this seasonal sequence of species which they found in their long series of tow-nettings at Port Erin, made an important suggestion as to its cause.
“It is known that some bacteria are incapable of producing their typical effects (say in fixing elementary nitrogen from its solution in sea water) if they are present in pure culture. In order to function effectively they must be associated with some other organism which, by itself, cannot produce the effect in question. Probably such symbiotic relationships may exist on the great scale in the sea. The work of Allen and Nelson (1910) on the artificial culture of diatoms suggests this. In mixed cultures there is always a certain succession of species, one attaining its maximum when another has ceased actively to reproduce. The succession of diatom species during the period of the spring growth suggests that something of the same kind occurs in the sea.”
A similar effect has, of course, now been demonstrated by Sir Alexander Fleming’s great discovery that moulds such as Penicillium produce substances which inhibit the growth of bacteria. In my hypothesis of animal exclusion (in Hardy and Gunther, 1935), which will be referred to again in a later chapter (see here), I have suggested that dense concentrations of planktonic plants may produce an effect in the water which is uncongenial to animal life and so account for the fact that animals are usually scarce in regions of great phytoplankton abundance. Dr. C. E. Lucas, my former pupil and colleague, now Director of the Scottish Fishery Laboratory at Aberdeen, has developed much further the idea of chemical interaction between organisms and stressed the possible importance of various substances given out into the water by different plants and animals as a result of their internal activities. Just as cells inside the body of an animal produce those various substances called hormones (or endocrines) which circulate in the blood stream to have profound effects on other parts of the body, so also may substances (ectocrines) be liberated from the body to have their effects on other organisms in an aquatic environment. The changed conditions set up in the water by one species may perhaps become both injurious to itself and at the same time more suitable to another kind which will follow it. Thus, among other interesting ideas, he gives strong support to this idea of seasonal succession: a chain of action, a conditioning and reconditioning of the water, as the year advances (Lucas, 1938, 1947 and 1956a).
Among the animals of the plankton there are also successive changes; particularly noticeable are the various broods of different species which follow one another, giving us in one month mainly adults and in another the young developing stages. The seasons are marked too by the throwing up into the plankton of the young larval stages of various bottom-living invertebrates, and also by the eggs and fry of different species of fish, all of which have their own distinct breeding times.
To return to more general considerations, it is interesting to compare the conditions as found in our waters at mid-summer with those in the surface waters of the tropics; we have the same heating up of the surface to form a discontinuity layer, but there it tends to be a permanent feature. In the open ocean in the tropics the phosphates and nitrates in the upper layers are thus reduced to a minimum all the year round and as a consequence the plankton of those regions is extraordinarily sparse compared with the more temperate or polar seas. This relative poverty of the tropical seas compared to our own was one of the surprising discoveries made by Victor Hensen’s German Plankton Expedition in 1889 and was at first disbelieved by many who thought, on false a priori grounds, that the warmer tropical seas must be richer in life than our own or the cold polar regions. A tow-netting in the tropical ocean may yield many more species than one in our own waters, but the total quantity of life is very much less; when we glance at a tropical sample at first sight nearly every specimen seems a different kind, whereas in one from our own waters there will be thousands of representatives of the same species. Plankton at certain places in the tropics, however, can be remarkably rich; this happens when deeper water with a supply of nutritive salts comes welling up into the sun-lit zones as against the coast or where a submarine bank comes near the surface and gives rise to disturbed water conditions.
The upwelling of water rich in phosphates and nitrates may well be very important in producing a more prolific phytoplankton in our own waters. There can be no doubt that the abundance of fish-food on the sea-bed which makes the Dogger Bank so renowned as a fishing ground, is due to the heavy rain of plankton showered upon it from above; there can also be little doubt that this rich phytoplankton in the surface layers is in turn produced by the upwelling of the richer phosphates and nitrates from below as the Atlantic inflow into the North Sea meets this large submarine bank set across its path (Graham, 1938).
A prolonged off-shore wind may have the effect of producing a heavy crop of plankton near the coast: it pushes the surface water away from the land so that its place has to be taken by water from below which wells up near the coast and thus again brings the desired nutritive salts up into the sunlit upper layers.
There are indications that at times other minor constituents of sea-water may have an effect upon phytoplankton. There is some experimental evidence that organic salts of iron and manganese will stimulate phytoplankton production; and it is suggested that such salts carried out by the drainage from the land may lead to an earlier outburst of reproductive activity among planktonic diatoms in coastal waters than among those further out to sea. Much information about these minor constituents will be found summarised by Dr. H. W. Harvey (1942, 1955) who has himself done so much of the experimental work.
For a long time there has been evidence that suggests that there is in the sea some trace substance at present unknown which is necessary before life can exist—some substance rather like the vitamins in our diet. Artificial sea-water has been made up to contain precisely the same proportions of chemicals that are known to be present in natural sea-water by the most exact analysis; yet planktonic plants will not grow in it unless about 1% of natural sea-water has been added to it (Allen, 1914). Quite recently the vitamin B12 (Cobalamin) has been shown to be necessary for the growth of several marine flagellates and a diatom (Droop, 1954 and ’55) and its presence in natural sea water has now been demonstrated.