
Flowers of the Coast
Although there is much which is obscure about the transpiration process, it has two important effects. Firstly, it maintains a constant flow of water from root to leaf through the wood of the plant, bringing with it also small quantities of dissolved salts which are essential for the plant’s growth. Secondly, it tends to reduce the temperature of the leaf when it is exposed to the heat of the sun. It is a well-known fact that when a liquid is changed into vapour, energy (latent heat) has to be expended. This heat is derived from the air immediately in contact with the surface of the leaf and in this way the leaf itself is cooled. In hot climates and in dry habitats this result may be important. The chief danger with xerophytes, and to a lesser extent with halophytes, is that the loss of water by transpiration may be so rapid that it cannot be replaced from the scanty supply of water available at their roots. Many plants belonging to both these classes are therefore equipped with devices to check excessive transpiration, and some of these will now be described.
TRANSPIRATION-CHECKS
OR DEVICES FOR REDUCING TRANSPIRATION
The development of a thick cuticle or outer skin on the leaves is the simplest and most frequently adopted method for the reduction of transpiration. The leathery feel of the leaves produced by many seaside plants is a characteristic which can hardly be overlooked, though the development of thick cuticles is by no means confined to coastal plants. In some cases this thickening is supplemented by the secretion of wax on the leaf surface, as in the case of the sea-holly (Eryngium maritimum) (Pl. 1). These protective layers have the effect of confining the evaporation of water entirely to the stomata, for in their absence a considerable amount of water is lost through the rest of the surface. Fig. 3 shows diagrammatically a transverse section round a stoma of a leaf with a thin cuticle (a) and a similar section from a leaf with a thick cuticle (b). It will often be noticed that the thickness of the cuticle varies considerably amongst individuals of the same species, according to the habitat in which they are growing. The leaves of the scarlet pimpernel (Anagallis arvensis) (Pl. 2b), for instance, become thick and leathery when it is growing on bare sand amongst dunes, although under normal conditions in garden soil they are soft and slender.
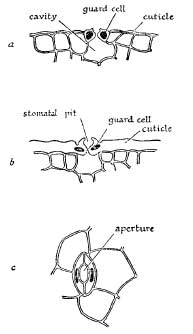
FIG. 3.—Types of stomata: a. Transverse section of leaf with a thin cuticle: b. Transverse section of leaf with a thick cuticle, showing a sunken stoma; c. Surface view of a stoma.
In many plants the stomata are protected by being placed in grooves or hollows sunk well below the surface of the leaf (Fig. 3(b)). In the dune grasses, marram-grass (Ammophila arenaria) and sea lyme-grass (Elymus arenarius), the stomata are mostly confined to the bottom and sides of the deep grooves in their leaves. This protection is much improved by the tendency of the leaves to roll up into a narrow tube in dry weather, which has the effect of maintaining a layer of air, largely saturated with water-vapour, between the stomata and the outside air, and thus reducing evaporation. The manner in which air is enclosed when the leaf rolls up is clearly shown in Fig. 4, and the corrugated inner (i.e. upper) surface is due to the deep grooves along which the stomata are scattered. The outer (i.e. under) surface is furnished with a thick cuticle and is devoid of stomata. This habit of rolling the leaf under dry conditions is shared by many plants, and is a good example of the way they can adjust themselves to variations in their water-supply. When water is plentiful, the blade opens out and becomes flat, thus exposing a greater surface for transpiration. The fresh appearance of marram-grass on open sand-dunes after abundant rain is quite distinct from its parched look after a long period of dry weather, and on closer inspection will be found to be due to the unfolding of its leaves.
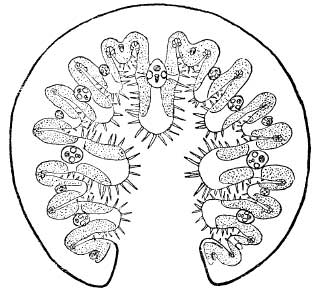
FIG. 4.—Transverse section of marram-grass leaf when rolled (from Fritsch & Salisbury, 1946).
Another common way in which the stomata are protected is by the growth of hairs on the surface of the leaf. These are often associated with sunken stomata and are very effective in maintaining a damp atmosphere round the opening, since moisture tends to condense on them. The stiff hairs protecting the furrows on the upper surface of the marram-grass leaf will be noticed in Fig. 4. Many seaside plants have hairy leaves, and some are covered with a thick down. The yellow horned poppy (Glaucium flavum) (Pl. IX), the sea stock (Matthiola sinuata) and the buck’s horn plantain (Plantago coronopus) (Pl. XXXVI) are good examples of coastal plants with hairy leaves, while the leaves of sea-wormwood (Artemisia maritima) (Pl. XXXI) and the tree-mallow (Lavatera arborea) are markedly downy. The characteristic silvery foliage of the sea-buckthorn (Hippophae rhamnoides) (Pl. XX) and sea-purslane (Halimione (Obione) portulacoides) (Pl. 16) is also due to scale-like (peltate) hairs covering the surface of the leaves. These hairs are usually dead when the leaf is mature, and contain only air. Apart from aiding the retention of moist air near the surface, they reflect much of the sun’s heat. Some leaves possess simple unbranched hairs, but those on many others are branched and occur in very different forms. Some typical covering hairs from the leaves of coastal plants are shown in Fig. 5. Like the thickness of the cuticle, the degree of hairiness shown by individuals of the same species often varies with availability of the water-supply in the habitat. Thus the sand-dune form of silverweed (Potentilla anserina) commonly shows a thick felting of silvery hairs on the upper surface of its leaves, as well as on the lower.
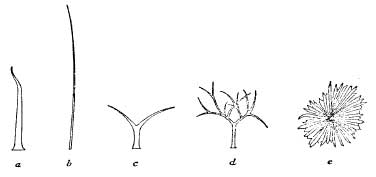
FIG. 5.—Typical covering-hairs on various leaves: a. Plantago coronopus; b. Cynodon dactylon; c. Erophila verna; d. Matthiola sinuata; e. Hippophae rhamnoides.
Still another way in which relatively damp air is maintained over the surface of the leaves is by the plant adopting a dense mat habit, so that the transpiring surfaces of the leaves are kept in close contact with each other. Alpine plants often mass their foliage in this way, but amongst coastal plants thrift (Armeria maritima) provides one of the best examples, since its habit varies considerably with the place in which it is growing. Thus the close rosette form is typical when it is growing on rocky cliffs and other dry habitats, or when it is heavily grazed, whilst with a better water-supply it assumes a much more open habit (Fig. 6). Many sand-dune plants spend most of the year in the form of a rosette, only sending up a vertical stem during the flowering season. In this way, only the upper surface of the leaf is exposed to the wind, the under surface being kept closely pressed against the surface of the sand, where it is fully protected from both sun and wind and consequently remains cool and moist.
Transpiration is discouraged in a large number of widely differing plants by a reduction in the actual surface of the leaves. Many conifers furnish examples of this; pines have needle-shaped leaves, and cypresses have scale-like leaves, which are closely pressed to the stem over part of their surface. Among coastal plants, tamarisk (Tamarix gallica) (Pl. 8), now a well-established alien in Britain, has numerous little scale-like leaves, and in the glassworts (Salicornia) the rudimentary leaves are only just visible as tiny scales which are firmly attached to the joints of the succulent stems (Fig. 9(b)). In some plants the same result is achieved by the leaves taking the form of spines. Gorse (Ulex spp.) is the best-known example in this country, but in desert regions the majority of the xerophytic plants show this modification, the Cacti being a familiar case. Our native xerophytes more frequently develop spiny margins to their leaves, thistles furnishing the obvious example. The most striking seaside plant to show this development is the sea-holly (Pl. 1), though the leaves of the prickly saltwort (Salsola kali) (Pl. I) also terminate in stout spines. The tendency to form woody tissue in the form of spines appears to be closely related to a shortage in the water-supply. A number of plants which produce spines when growing in dry habitats do not possess any when moisture is abundant.
Occasionally the function of the leaf is taken over by specially modified branches known as “cladodes.” The only coastal plant exhibiting this modification is the wild asparagus (Asparagus prostratus), a rare plant found on sandy shores in a few localities only in this country. If the familiar feathery foliage of the garden asparagus is examined, it will be seen to consist of tufts of short leaf-like branches arising from the axils of minute scaly leaves (Fig. 7). It is difficult to see exactly what advantage a plant can gain from the substitution of a leaf-like stem for an ordinary leaf—possibly the tissue of the cladode is more resistant to shrinkage when the plant is suffering from a shortage of water.
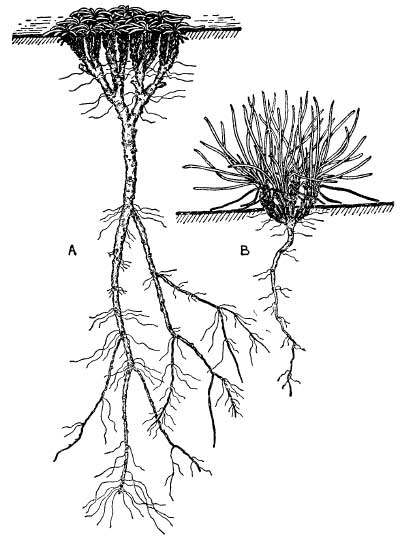
FIG. 6.—Different forms adopted by thrift: a. Rosette form under grazing or in dry ground; b. More diffuse habit when protected from grazing and with a good water-supply (from Tansley after Yapp, 1917).
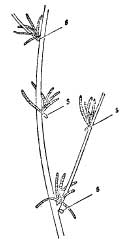
FIG. 7.—Part of a branch of asparagus, showing cladodes and scale-leaves (s).
Quite apart from these permanent alterations in leaf-form, the shape and size of the leaves of many common plants vary greatly with the conditions under which they grow. For example, the first leaves of the red-fruited dandelion (Taraxacum laevigatum), when growing in a moist hollow among sand-dunes, are often quite entire (i.e. with smooth edges); later in the season, when the sand has become dry, it produces the more familiar deeply divided leaves with a much smaller surface-area (Fig. 8). Most of the common inland plants found on sand-dunes possess smaller leaves than when they grow on more hospitable ground. Nor must we forget that the semi-prostrate form so frequently adopted by dune-plants is still another method by which excessive transpiration can be reduced, since every extra inch in height exposes the plant more to the desiccating action of the strong winds.
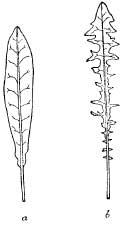
FIG. 8.—Different leaf-forms of the red-fruited dandelion: a. Young leaf from a plant growing with abundant moisture. b. Leaf from a plant growing on dry sand.
It will be clear from what has been said that many plants are capable of modifying their normal form when growing in dry habitats. Of the various transpiration-checks which have been described, undoubtedly the development of a thick cuticle is the most frequent one employed by coastal plants. It possesses an added importance for plants inhabiting open sand-dunes in that it also protects them from possible injury caused by the sand being blown against them. Anyone who has done any botanising on exposed sand-dunes during a high wind will know how violent this bombardment can be!
It is important to point out, before we leave this subject, that it is only during periods of water-shortage that these mechanisms for reducing transpiration become important. Recent research has shown that xerophytes transpire during wet spells at least as much as, and often more than, ordinary plants. In those cases where the transpiration-rate becomes unusually high, it may be related to the necessity for rapid growth and carbon assimilation during the infrequent wet periods. What really characterises a xerophyte is that it can, if need be, decrease its transpiration-rate to a minimum when living under drought conditions. In addition, the actual protoplasm (living matter) seems able to withstand desiccation to an unusual extent.
SUCCULENCE
Some xerophytes employ quite a different method to provide against water shortage, though it is often found in combination with the leaf-modifications already described. It will be noticed that many plants growing in dry places have a fleshy or succulent appearance. This is due to the development of large colourless cells, known collectively as “aqueous tissue,” which are employed for storing water. This is usually confined to the leaves as in the stonecrops (Sedum spp.) (Pl. 14) or the sea-spurge (Euphorbia paralias) (Pl. XIX), but sometimes the whole stem is succulent as in the glassworts (Salicornia spp.) (Fig. 9(b)) or the familiar Cacti, the leaves in these cases being reduced to mere scales or spines. As a rule these cells occupy the centre of the leaf or stem, and the green cells which are used for photosynthesis occur nearer the edges. In dry weather, as water is gradually lost by transpiration or by its passage into the green cells, the water-holding cells shrink; when the water-supply improves, they expand once more. They function, in fact, as water-storage cisterns for use by the plant in times of drought. Desert succulents, which often possess extremely thick cuticles to reduce transpiration, can exist for prolonged periods without an external supply of water, during which they gradually shrivel until they can replace their internal water supplies when the rain comes.
It is rather surprising that halophytes should form the largest class of plants exhibiting succulence in this country. It has already been pointed out that the chief characteristics of this group are that they can exert sufficiently large osmotic pressures to withdraw water from a soil which is saturated with sea-water, and that the protoplasm forming their cells is not injured by exposure to salt solutions. Under normal conditions, therefore, they should not encounter much trouble with their water-supplies, and it is difficult to understand why they should develop aqueous tissue so extensively. It is possible that they draw on their internal reserves of water when the concentration of salt in the soil alters too rapidly for them to accommodate their osmotic pressure to it, but this can hardly account for such a widespread characteristic. Furthermore, it has been shown that halophytes do not, in fact, withstand drought like succulent xerophytes, but actually wither quite rapidly.
The most likely explanation is that the similarity in appearance of succulent xerophytes and halophytes is largely accidental. There is considerable evidence to suggest that the latter become succulent as a result of some chemical effect associated with salt, probably with the chlorine rather than the sodium part of it (salt is a simple compound between these two elements). All plants when growing in a saline soil absorb some salt, for the cell-walls of the root-hairs never function as “perfect” semipermeable membranes, but allow a certain amount of the dissolved substances to pass through them. This is, of course, true of all types of plant, for otherwise they would be unable to obtain the small quantities of other mineral salts essential to their growth. Many halophytes, however, absorb very large amounts of common salt; the ash of some of them, like the glassworts, was formerly used on a large scale to provide soda for glass-making, and certain plants, as for example thrift, actually excrete surplus salt from the glands on their leaves. That the development of succulent leaves is closely connected with the absorption of salt is borne out by the behaviour of many non-halophytes when they grow in places exposed to sea-water. Many inland plants found on open beaches or on cliffs within reach of sea-spray possess much more fleshy leaves than they have in their normal habitats; bird’s-foot trefoil (Lotus corniculatus), kidney vetch (Anthyllis vulneraria) and the greater knapweed (Centaurea scabiosa) are species which often show this effect. Some years ago I analysed the ash of certain inland plants which had been exposed to sea-spray in this way and found 13.5 per cent of salt in that of the kidney vetch. Evidence such as this points strongly to the conclusion that some chemical action connected with salt is the primary cause of succulence in halophytes, and there seems little reason to associate it with the problem of conserving water. The similarity between succulent xerophytes and halophytes is remarkable and we must leave it at that.
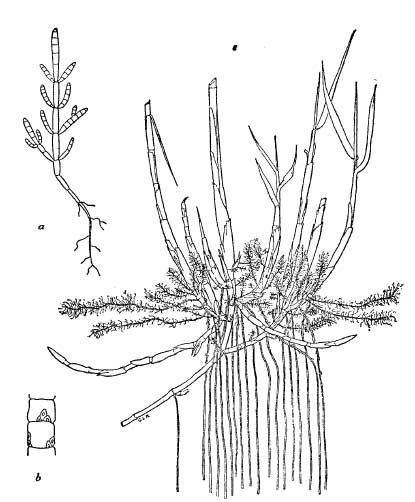
FIG. 9.—Comparison of root-systems of annual glasswort and rice-grass:-a. Small plant of annual glasswort; b. Two joints from stem of annual glasss wort enlarged to show leaf-scales; c. Base of a rice-grass plant showing vertical anchoring roots and horizontal feeding roots and stolons (Fig. c from Tansley after Oliver, 1926).
ROOT-SYSTEMS
The roots of coastal plants are very characteristic and present many points of interest. The majority of true halophytes (i.e. plants which normally grow where the soil-water is saline) possess very deep roots, generally markedly woody. Most of these plants are perennials and the chief value of their long roots in a salt-marsh is to enable them to secure a firm anchorage in relatively unstable mud. It also allows them to derive their main water supplies from regions where the concentration of salt is less variable than it is in the surface layers. Annual glasswort (Salicornia stricta) (Pl. XIII) possesses only quite short roots, and as a result is liable to become dislodged from the unstable mud if there is a strong tidal flow (Fig. 9(a)). This is in marked contrast to rice-grass (Spartina townsendii) (Pl. XIV), which occupies much the same position as a pioneer colonist in many of the south coast salt-marshes. This plant develops a most extensive root-system and becomes so firmly anchored in the mud that it can easily resist the strong currents produced by the ebb and flow of the tides (Fig. 9(c)). Its remarkable powers of spreading over soft mud and stabilising the surface are described more fully on page 71. Plants growing on sea-cliffs also develop very long roots, which serve the dual purpose of anchoring them firmly against uprooting by the violent winds encountered in these exposed places and of enabling them to tap deep-seated supplies of water. Well-established plants of thrift or samphire (Crithmum maritimum) (Pl. 10) frequently possess roots several feet long, which penetrate deeply into the crevices between the rocks.
Extensive root-systems are also a characteristic feature of xerophytes in all parts of the world. In sand-dunes and shingle this enables the plants to utilise the moisture which is always present some way below the surface (see here). In addition, the elaborate root-systems developed by many dune plants perform the important function of binding blown sand. All pioneer colonists on sand-dunes, and to a lesser extent those on mobile shingle, have to contend with the possibility of being periodically swamped by loose sand or shingle. Most of them have, in varying degrees, the ability to form fresh shoots easily when they are submerged in this way, and to grow up through this covering. Marram-grass easily outstrips all other plants in the vigour with which it can do this. When once established in loose sand, it soon produces a mass of underground runners from which new shoots continually spring. Where these new shoots occur, fresh adventitious roots are produced under them; as the stems and leaves become buried by sand, further shoots and leaves are produced at a higher level on the stem (Fig. 10). The thick tufts of leaves and young shoots are very stiff and offer a considerable obstruction to the wind, causing it to drop some of its load of sand round them. Provided the blown sand does not accumulate too rapidly over the plants, marram-grass can continue to grow upwards through many feet of sand. In this way dunes rising to considerable heights can be produced, their size depending on the supply of available sand and the strength of the prevailing winds. The whole interior of the dune remains closely penetrated by a mass of rhizomes (underground stems) and fine roots which bind it together. As these rhizomes and roots are constantly being renewed at higher levels, the lower ones gradually die off, but their dead remains persist in the lower regions of a dune for a long time and continue to exercise a stabilising effect on it (Pl. XXI).
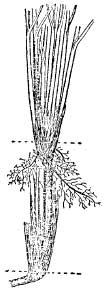
FIG. 10—Leaf production at different levels shown by marram-grass (from) Firistch & salisbury, 1946).
To a lesser extent the sea couch-grass (Agropyron junceiforme) can produce a similar result. This plant has much the same habit as ordinary couch-grass or twitch (Agropyron repens), which is an all too familiar agricultural weed. Like marram-grass, it produces a mass of rhizomes from which new shoots spring up at frequent intervals, but its runners tend to spread more rapidly in a horizontal direction than vertically when covered by loose sand. As a result, the dunes it produces are comparatively low compared with those formed by marram-grass. None the less, its powers of binding sand are considerable, and single plants have been shown to cause dunes as much as 20 feet across during a few years’ growth. The sea lyme-grass (Elymus arenarius) has a similar root-system and occasionally forms low dunes of the same type on certain parts of the coast.
Many other pioneer plants on shifting sand can accumulate small amounts of sand round them to form miniature dunes, provided they are sufficiently virile to shoot up again when they become buried. For example, the sea-sandwort (Honckenya (Arenaria) peploides) is a low-growing plant not more than a few inches high, but it possesses surprisingly extensive creeping roots and readily produces fresh shoots when it is covered. Even those common pioneers of sandy beaches, the sea-rocket (Cakile maritima) (Pl. VII) and the prickly saltwort (Pl. I), which are only annuals, can collect tiny dunes round their long trailing branched stems. Their dead remains usually persist for a considerable time in the winter and continue to hold the sand, although the principal agent here is the stem rather than the roots.
Marram-grass has relatively little stabilising effect on the surface sand, and it is the later colonists which establish themselves between the clumps that are responsible for its eventual consolidation. Notable amongst these are the sand-sedge (Carex arenaria) and the sand-fescue (Festuca rubra var: arenaria), both of which produce horizontally creeping roots just below the surface. Tufts of foliage arise from these runners at frequent intervals, and in the case of the former often appear spaced out along a nearly straight line for many feet, showing clearly the course of its immense roots (Pl. X).
Plants growing on shingle similarly have to endure periodical swamping by stones. Very unstable shingle is usually devoid of vegetation, but during storms those less mobile parts of the beach, which carry quite a large amount of vegetation, may also be disturbed. The shrubby seablite (Suaeda fruticosa) (Pl. XXIII), a local plant which is found on Chesil Bank and on some shingle beaches along the Norfolk coast, adapts itself in an interesting way to these conditions. When it grows in perfectly stable shingle it is a plant of erect habit, 3-4 feet high, but where the shingle is more mobile it assumes a quite different, semi-prostrate habit. Under the latter conditions, the stem is tilted forwards as shingle flows over it from above and the lower part becomes imbedded. New branching shoots then arise from the buried stem, and at the same time fresh tufts of roots are produced under each shoot. When growing on very unstable shingle banks, the original axis of many of these plants may become quite horizontal, and a complex system of prostrate branches, covered everywhere with fibrous roots, will be found in the form of a dense mat just beneath the surface. The aerial shoots from these extensive buried stems grow vertically upwards, usually to a height of a foot or two, but much of the elaborate underground structure is generally dead and already in the process of decay (Fig. 11).